Summary answer:
The rats in Séralini’s study had unrestricted access to food and water, but so did the rats in Monsanto’s 90-day studies on GM foods, and so do most humans in their daily lives. So this aspect of Séralini’s
Read More
Criticism: Sprague-Dawley rats get tumours when food intake is unrestricted
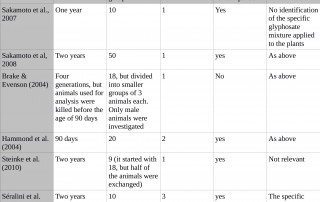
Criticism: Séralini used an insufficient number of controls
Summary answer:
Séralini’s control groups were the same size as each treatment dose group, in line with standard scientific practice. It is not good scientific practice to introduce extra irrelevant “reference” control groups, though Monsanto has routinely done this in
Read More
Criticism: No food intake data is presented, so we don’t know the dose of toxins ingested
Summary answer:
Séralini measured food intake more often than industry studies on GM foods and the absence of data in his published paper does not invalidate the findings observed. (more…)
Criticism: Toxic effects did not follow a linear dose-response pattern
Summary answer:
Many toxins, especially those that affect the hormonal system (these include Roundup), have nonlinear dose-response patterns. Scientists have published papers about nonlinear dose-response patterns since the 1990s, but industry and some risk assessment bodies cling to the outdated
Read More
Criticism: Outcomes were sex-specific, e.g. the majority of tumours were found in females
Summary answer:
Sex-specific toxic effects are well documented in the scientific literature, including in a study on Roundup toxicity in rats and in animal studies on GM foods. Such effects are to be expected when the hormonal system is involved.
Read More
Criticism: No mechanism for the effects observed has been established
Summary answer:
There is no requirement in any regulatory system to establish mechanism of action for a toxin before regulatory action can be taken and there is no burden of proof on scientists who find toxic effects to establish a
Read More
Criticism: There was no good reason for Séralini to test these doses of GM NK603 maize and Roundup
Summary answer:
Regarding NK603 maize, Séralini used the same two doses as Monsanto used in its 90-day study and added a third dose, which is necessary to draw any conclusions about dose response in relation to any harmful effects. Regarding
Read More
Criticism: Séralini’s study is inadequate and should be dismissed
Summary answer:
No study is perfect, but Séralini’s is far stronger than the 90-day studies carried out by industry for regulatory authorization of GM foods. It is unacceptable that these studies are accepted as proof of safety for GM foods
Read More
Double standards used to evaluate GMO safety tests
Source: Then, C. (2012). The European Food Safety Authority: Using double standards when assessing feeding studies. Testbiotech. http://www.testbiotech.de/node/725
Critics answered: Conclusion
At present there are no internationally agreed mandatory guidelines on how GM crop animal feeding safety trials should be conducted. Although OECD guidelines for chemical safety testing are a reasonable staring point, there is an urgent need for internationally
Read More