Summary answer:
Séralini’s control groups were the same size as each treatment dose group, in line with standard scientific practice. It is not good scientific practice to introduce extra irrelevant “reference” control groups, though Monsanto has routinely done this in its tests on GM foods. This practice only introduces greater variability and data “noise” which can hide any toxic effects of the genetic modification.
Detailed answer:
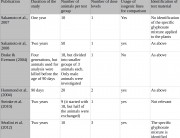
Critics claim that Séralini used inadequate or too-small control groups.1 But Séralini’s experiment followed standard scientific practice regarding the number of controls and how they were used. The rats in Séralini’s experiment were divided into ten groups of 20 animals each (ten male and ten female), with nine of the groups exposed to different doses of Roundup, or NK603, or Roundup and NK603 combined.
In line with standard scientific practice, the control group was matched in size to the experimental groups. Each experimental group was ten animals of each sex (20 animals per treatment dose group). Therefore the control group not exposed to NK603 or Roundup was also ten animals of each sex (20 animals altogether). Each treatment group of 20 animals was compared separately with the control group of 20 animals. This control group was therefore a valid control group for each of the treatment groups.
Industry tests on GM foods often introduce additional irrelevant “reference” control groups, fed a variety of different diets. These consist of non-GM but genetically different (non-isogenic) varieties of the GM food being tested. Adding these extra control groups also means that there are far more animals in the overall control group against which each treatment group is compared.2 3 4
Using additional control groups is not only non-standard – it is bad scientific practice. Even the OECD guideline 116 for chronic toxicity and carcinogenicity tests says it is “rarely necessary”.5 One control group is the norm. Extra control groups serve only to increase rather than decrease variables in the experiment. They raise background baseline reference measurement levels – in other words, they add data “noise” – which can mask any effects arising from the consumption of the GM feed under test.
Ironically, Séralini has been criticised for NOT including such irrelevant control groups in his experiment – for example, by Wendy Harwood, senior scientist at the John Innes Centre in the UK,1 which develops GM traits for industry.
Séralini’s team responded that in controlled scientific experiments, we test one variable at a time.6 This means that any differences found in the treatment groups are likely to have arisen from the substance being tested – in this case, the GM maize NK603, or Roundup, or a combination of the two, according to the respective treatment groups – and not from some irrelevant factor.
References :
1. Science Media Centre. Expert reaction to GM maize causing tumours in rats [press release]. 19 September 2012. http://www.sciencemediacentre.org/pages/press_releases/12-09-19_gm_maize_rats_tumours.htm
2. Hammond B, Dudek R, Lemen J, Nemeth M. Results of a 13 week safety assurance study with rats fed grain from glyphosate tolerant corn. Food Chem Toxicol. Jun 2004; 42(6): 1003-1014.
3. Hammond B, Lemen J, Dudek R, et al. Results of a 90-day safety assurance study with rats fed grain from corn rootworm-protected corn. Food Chem Toxicol. Feb 2006; 44(2): 147-160.
4. Hammond BG, Dudek R, Lemen JK, Nemeth MA. Results of a 90-day safety assurance study with rats fed grain from corn borer-protected corn. Food Chem Toxicol. Jul 2006; 44(7): 1092-1099.
5. Organisation for Economic Cooperation and Development (OECD). Guidance document 116 on the conduct and design of chronic toxicity and carcinogenicity studies, supporting test guidelines 451, 452 and 453: 2nd edition: Environment directorate joint meeting of the chemicals committee and the working party on chemicals, pesticides and biotechnology. 13 April 2012.
6. CRIIGEN. FAQ – From CRIIGEN research team September 2012. http://www.criigen.org/SiteEn/index.php?option=com_content&task=view&id=368&Itemid=1
Sources of criticism:
Science Media Centre “experts”, notably Anthony Trewavas
http://www.sciencemediacentre.org/expert-reaction-to-gm-maize-causing-tumours-in-rats/
European Food Safety Authority
http://www.efsa.europa.eu/en/efsajournal/pub/2910.htm
http://www.efsa.europa.eu/en/efsajournal/pub/2986.htm
Haut Conseil des Biotechnologies (HCB), France
http://www.hautconseildesbiotechnologies.fr/IMG/pdf/Etude_Seralini_Avis_CS_HCB_121019.pdf