Summary answer:
This criticism hinges on the incorrect assumption that Séralini’s study was intended to be a carcinogenicity study. The critics say that Séralini used too few rats of a strain prone to tumours, so the tumours seen may have occurred spontaneously and no conclusions can be drawn. But Séralini’s study was a chronic toxicity study, not a carcinogenicity study. The increase in tumour incidence was a surprise outcome. The logical response to the findings is not to dismiss them but to follow up with a full-scale carcinogenicity study on GM NK603 maize and Roundup.
Detailed answer:
This criticism hinges on the incorrect assumption that Séralini’s study was intended to be a carcinogenicity study. The critics say that Séralini used too few rats, of a strain prone to tumours, so the tumours seen may have occurred spontaneously and no conclusions can be drawn.
ButSéralini designed his study as a chronic toxicity study, not a carcinogenicity study. The increase in tumour incidence was a surprise outcome. No existing data from the developer of NK603 maize, Monsanto, or elsewhere indicated that NK603 maize or Roundup were carcinogenic. Unless Séralini had employed Mystic Meg as his adviser, there was no reason for him to embark on a carcinogenicity study. A dedicated carcinogenicity study would have involved using five times more animals and would have made the study virtually impossible to afford by an independent academic research group.
The omission in this case is not Séralini’s but that of industry and regulators. Industry has failed to carry out carcinogenicity studies on GMOs or complete herbicide formulations like Roundup before releasing them onto world markets, and regulators have failed to require them.
The aim of the chronic toxicity study design employed by Séralini was to follow up the initial signs of liver and kidney toxicity that his team had previously found in their re-analysis of the data from Monsanto’s 90-day study on NK603 maize.1
Because his study had too few animals to comply with standard carcinogenicity protocols set by the Organisation for Economic Cooperation and Development (OECD) and other bodies, he did not do a statistical analysis on the findings related to changes in tumour or mortality incidence. A dedicated carcinogenicity study using larger numbers of animals would have to be carried out to enable such analyses.
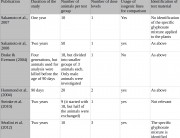
Séralini’s study should be evaluated on its own terms: as the most detailed and in-depth long-term toxicity study that has ever been done on a GM food and its associated herbicide. Séralini measured more effects over a longer period than any industry study on a GMO performed for regulatory authorization, and analyzed the same number of animals as Monsanto in its 90-day studies on GMOs.23 4 Furthermore, this is the first study to enable the effects of a GM food to be distinguished from those of its associated pesticide.
There is no more point in criticizing Séralini’s study for not being a carcinogenicity study than there is in criticizing Monsanto’s 90-day feeding trials on GM foods for not being carcinogenicity studies, or in criticizing an apple for not being a banana. It is simply irrelevant. What is clear is that industry must carry out dedicated carcinogenicity studies on all its GM products and associated herbicides before releasing them into our food supply.
References:
1. de Vendomois JS, Roullier F, Cellier D, Séralini GE. A comparison of the effects of three GM corn varieties on mammalian health. Int J Biol Sci. 2009; 5(7): 706–726.
2. Hammond B, Dudek R, Lemen J, Nemeth M. Results of a 13 week safety assurance study with rats fed grain from glyphosate tolerant corn. Food Chem Toxicol. Jun 2004; 42(6): 1003-1014.
3. Hammond B, Lemen J, Dudek R, et al. Results of a 90-day safety assurance study with rats fed grain from corn rootworm-protected corn. Food Chem Toxicol. Feb 2006; 44(2): 147-160.
4. Hammond BG, Dudek R, Lemen JK, Nemeth MA. Results of a 90-day safety assurance study with rats fed grain from corn borer-protected corn. Food Chem Toxicol. Jul 2006; 44(7): 1092-1099.
Sources of Criticism:
Science Media Centre “experts”:
http://www.sciencemediacentre.org/expert-reaction-to-gm-maize-causing-tumours-in-rats/
Monsanto:
http://www.monsanto.com/products/Documents/ProductSafety/seralini-sept-2012-monsanto-comments.pdf
European Food Safety Authority:
http://www.efsa.europa.eu/en/efsajournal/pub/2910.htm
http://www.efsa.europa.eu/en/efsajournal/pub/2986.htm
BfR (German federal institute for risk assessment)