Summary answer:
The Sprague-Dawley (SD) rat strain that Séralini used is also used in long-term 2-year toxicity and carcinogenicity studies by industry and academic scientists, as well as in 90-day studies on GMOs. If this was the wrong type of rat for Séralini to use, it was the wrong rat in all these other studies, and market authorizations for the thousands of chemicals and GM foods that were authorized on the basis of these studies should be revoked.
Detailed answer:
Critics say that the Sprague-Dawley (SD) strain of rat that Séralini used is naturally prone to developing tumours, so the increased tumour incidence found in the rats exposed to NK603 maize and Roundup may have been “spontaneous” – that is, they would have happened even without NK603 maize and Roundup. They conclude that Séralini’s tumour findings are meaningless.1
However, the SD rat is a standard choice for long-term (2-year +) studies for tumour-causing and carcinogenic effects by independent and industry-sponsored researchers.2 3 4 5 6 The National Toxicology Program in the US uses the same SD rat from the same source as Séralini’s rats (Harlan) for its long-term 2-year carcinogenicity and toxicology studies.7 None of these researchers or research programmes has been challenged over their use of SD rats.
“An absurd argument” – researcher
In a newspaper interview, the Swiss scientist Dr Angelika Hilbeck of the Swiss Federal Institute of Technology replied to the argument that Séralini chose a cancer-prone rat strain:
“This is an absurd argument. Séralini chose the same strain of rat as Monsanto. Do we really think that a substance should be tested on an animal that is not sensitive to it? With these defamations they wanted to distract us from the fact that Séralini used the same methodology as Monsanto. Because if you take Séralini seriously as a researcher, you have to take seriously his study and the comparison with Monsanto’s study. That would put into question Monsanto’s study and hence the approval of GM maize.”
Monsanto and other manufacturers of glyphosate, the main chemical ingredient of Roundup herbicide, used the SD rat in their two-year carcinogenicity and multigenerational reproductive toxicity studies that form the basis of the EU authorization of glyphosate.8 9
If the SD rat was the wrong rat for Séralini to use, it was also the wrong rat for all these other studies. So market authorizations for the thousands of chemicals and GM foods that were granted on the basis of these studies – including glyphosate – should be revoked.
The SD rat is also often used by industry in its 90-day tests on GMOs submitted to gain regulatory authorization. This includes Monsanto’s 90-day test on NK603 maize.10
Séralini correctly used the same rat strain that Monsanto used, in order to make his study comparable to Monsanto’s. This is in line with the recommendation of the Organisation for Economic Cooperation and Development (OECD) in its chronic toxicity protocol 453.11 The OECD says that for a chronic toxicity study, the same rat that was used in the 90-day test should be used. If Séralini had used a different strain, he would undoubtedly have been criticized for doing so and thus making his study not comparable with Monsanto’s 90-day test.
Is the SD rat abnormally prone to developing tumours?
The SD rat is about as prone to developing tumours as humans living in industrialized countries. Researchers view it as an excellent human-equivalent model for tumour-causing and cancer-causing effects.12
This includes the fact that in rats, as in humans, the number of tumours increases with age.12 Far from muddying the picture, as critics of Séralini charge, the fact that old rats get more tumours accurately reflects the reality of human ageing.
Background rate of tumours does not matter
The background rate of tumours in the strain of rat that Séralini used does not matter and does not invalidate his findings, as explained by Judy Carman, associate professor at Flinders University School of the Environment and director, Institute of Health and Environmental Research, Australia.
Dr Carman said:
“Séralini undertook a properly controlled experiment. This means that he used a control group that was not given any treatment. This control group tells you the background level of tumours in that type of rat. In science, you compare this background rate to the tumours you see in groups that you have ‘treated’ in some way. In Séralini’s case, the treatment groups were treated with GM NK603 maize and Roundup herbicide, separately and together.
“The aim is to see if the treatment increases the amount of tumours above the background rate. The increase is measured using something called the ‘relative risk’. For example, if a treatment results in twice as many tumours as the control group, then you say that the treatment has a relative risk of 2.
“Science is done this way because we know that there are background levels of tumours in animals and people. For example, you have a small risk of getting lung cancer even if you do not smoke. But you have a much higher risk of getting lung cancer if you do smoke. In fact, you have about a 12-fold higher risk of getting cancer if you smoke than if you do not smoke, so the relative risk here would be described as 12 for smoking and lung cancer.
“The rats that Séralini used may or may not have a high background level of getting tumours. It does not matter. The fact is, rats in treatment groups had a higher chance of getting tumours than rats than the control rats that did not get the treatment. Saying that the results were invalidated because the control rats had a higher background rate of tumours is as absurd as saying that smoking cannot cause lung cancer in an ethnic group if that group has a naturally-occurring higher background rate of lung cancer.”
In Séralini’s study, all treatments in both sexes increased large tumour incidence 2-3-fold in comparison to controls within the experiment.
By the beginning of the 24th month, 50-80% of female animals had developed tumours in all treated groups, with up to 3 tumours per animal. In the control group, in contrast, only 30% of the rats had tumours.
The most valid control for any experiment is the concurrent control – the control group within the experiment. However, industry and regulators often use “historical control data” from a variety of other experiments to evaluate the findings in any one experiment – generally to dismiss findings of toxic effects and to claim that the substance or product is safe.
Use of historical control data is bad scientific practice unless the comparability of each data point is established. But since it is common in the field of industry/regulatory science, Séralini briefly and in a summary statement evaluated his findings against published historical control data on the SD rat.
Séralini found that the treatments in his experiments increased the incidence of mammary tumours 2-3-fold in comparison to spontaneous tumour rates in the same SD strain from the same supplier (Harlan),13 and 3-fold in comparison to the largest study with 1329 SD female rats.14 In addition, tumours in Séralini’s treatment groups developed earlier and faster than in controls. This suggests that these tumours had a different biological basis from those arising spontaneously.
References:
1. Science Media Centre. Expert reaction to GM maize causing tumours in rats [press release]. 19 September 2012. http://www.sciencemediacentre.org/pages/press_releases/12-09-19_gm_maize_rats_tumours.htm
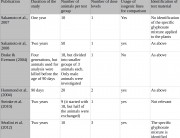
2. Voss C, Zerban H, Bannasch P, Berger MR. Lifelong exposure to di-(2-ethylhexyl)-phthalate induces tumors in liver and testes of Sprague-Dawley rats. Toxicology. Jan 31 2005; 206(3): 359-371.
3. Hack R, Ebert E, Leist KH. Chronic toxicity and carcinogenicity studies with the insecticide endosulfan in rats and mice. Food Chem Toxicol. Nov 1995; 33(11): 941-950.
4. Klimisch HJ, Deckardt K, Gembardt C, Hildebrand B, Kuttler K, Roe FJ. Long-term inhalation toxicity of N-vinylpyrrolidone-2 vapours. Studies in rats. Food Chem Toxicol. Oct-Nov 1997; 35(10-11): 1041-1060.
5. Gamez R, Noa M, Mas R, et al. Long-term carcinogenicity of D-003, a mixture of high molecular weight acids from sugarcane wax, in Sprague Dawley rats: a 24 months study. Food Chem Toxicol. Dec 2007; 45(12): 2352-2358.
6. Soffritti M, Belpoggi F, Degli Esposti D, Lambertini L. Results of a long-term carcinogenicity bioassay on Sprague-Dawley rats exposed to sodium arsenite administered in drinking water. Ann N Y Acad Sci. Sep 2006; 1076: 578-591.
7. National Toxicology Program. Toxicology/Carcinogenicity. 2012. http://ntp.niehs.nih.gov/?objectid=72015DAF-BDB7-CEBA-F9A7F9CAA57DD7F5
8. Rapporteur member state Germany. Monograph on glyphosate. Vol 3-1 Glyphosat 05. German Federal Agency for Consumer Protection and Food Safety (BVL). 1998. http://earthopensource.org/files/pdfs/Roundup-and-birth-defects/VOLUME3-1_GLYPHOSAT_05.PDF
9. Rapporteur member state Germany. Monograph on glyphosate. Vol 3-1 Glyphosat 04. German Federal Agency for Consumer Protection and Food Safety (BVL). 1998. http://earthopensource.org/files/pdfs/Roundup-and-birth-defects/VOLUME3-1_GLYPHOSAT_04.PDF
10. Hammond B, Dudek R, Lemen J, Nemeth M. Results of a 13 week safety assurance study with rats fed grain from glyphosate tolerant corn. Food Chem Toxicol. Jun 2004; 42(6): 1003-1014.
11. Organisation for Economic Cooperation and Development (OECD). OECD guideline no. 453 for the testing of chemicals: Combined chronic toxicity/carcinogenicity: Adopted 7 September 2009. 2009.
12. Soffritti M, Belpoggi F, Degli Esposti D. Cancer prevention: The lesson from the lab. In: Biasco G, Tanneberger S, eds. Cancer Medicine at the Dawn of the 21st Century: The view from Bologna. Bologna: Bononia University Press; 2006:49–64.
13. Brix AE, Nyska A, Haseman JK, Sells DM, Jokinen MP, Walker NJ. Incidences of selected lesions in control female Harlan Sprague-Dawley rats from two-year studies performed by the National Toxicology Program. Toxicol Pathol. 2005; 33(4): 477-483.
14. Chandra M, Riley MG, Johnson DE. Spontaneous neoplasms in aged Sprague-Dawley rats. Arch Toxicol. 1992; 66(7): 496-502.
Sources of criticism:
Science Media Centre “experts”, notably Tom Sanders
http://www.sciencemediacentre.org/expert-reaction-to-gm-maize-causing-tumours-in-rats/
European Food Safety Authority
http://www.efsa.europa.eu/en/efsajournal/pub/2910.htm
http://www.efsa.europa.eu/en/efsajournal/pub/2986.htm
Science writer Declan Butler, writing in Nature journal
http://www.nature.com/news/hyped-gm-maize-study-faces-growing-scrutiny-1.11566
Weedcontrolfreaks.com
http://weedcontrolfreaks.com/2012/09/why-i-think-the-seralini-gm-feeding-trial-is-bogus/


















Did treatments and controls rats have the same age? Is it possible that controls showed lower percentage of cancer just because they were younger than treatments? Thanks