Summary answer:
Séralini has been criticized on both these grounds, though they contradict one another. In fact, Monsanto, not Séralini, is guilty of conducting studies with a pre-determined outcome. Also, the GM authorization system is inherently biased, in that industry tests its own products for safety. Séralini’s study was an open-ended chronic toxicity study, which examines a variety of effects. He did not expect to find an increase in tumour incidence, so he did not design his study as a carcinogenicity study – for which he has also been criticized!
Detailed answer:
Séralini has been criticized on both these grounds, though they contradict one another.
Journalist Michael Hiltzik wrote in the Los Angeles Times, “The chief overall criticism of his experiment is that it seemed designed to prove a specific conclusion, rather than objectively test a hypothesis”, since Séralini is “a campaigner against genetically modified foods”.1
But the European Food Safety Authority’s (EFSA) criticism of the study was the opposite: “The study objectives are unclear.”2
Perhaps EFSA would have been happy if Séralini had followed the lead of Monsanto in its 90-day study on the same GM maize that Séralini tested. Monsanto used a weak study protocol, which included analyzing blood and urine chemistry in only ten out of 20 animals in each test group and using two doses instead of the three recommended by the OECD. Nevertheless, the Monsanto authors called their study a “safety assurance study” and concluded that the maize was as “safe” as non-GM maize.3
Monsanto did what Hiltzik accused Séralini of doing: it designed its study to prove a specific conclusion, rather than to objectively test a hypothesis. Oddly, however, Hiltzik does not accuse Monsanto, but only Séralini.
In fact, Monsanto’s data did not support the study’s conclusion of safety or its upbeat title. The data was later re-analyzed by Séralini’s team, who concluded that it revealed signs of liver and kidney toxicity.4
Séralini designed his new study to follow up Monsanto’s 90-day study and find out what happened to these initial signs of toxicity. He wanted to find out if they were biologically irrelevant, as Monsanto3 and EFSA5 claimed, or if they developed into disease or led to premature death.
Séralini said in the introduction to his study that while GM companies have generally adapted to their own design the 90-day OECD protocol no. 408 for testing chemicals,
“We have explored more parameters and more frequently than recommended in this [OECD] standard … in a long-term experiment. This allowed us to follow in detail potential health effects and their possible origins due to the direct or indirect consequences of the genetic modification itself in GMOs, or due to the formulated herbicide mixture used on GMOs (and not glyphosate alone), or both.”
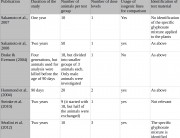
This open-ended approach is suited to a chronic toxicity study. The study design allowed the researchers to examine a broad range of endpoints (effects). The endpoints are listed in the study (Table 1).6 They include a range of blood and urine measurements unprecedented in breadth and well in excess of the measurements carried out by industry.
The researchers also performed detailed histopathology examinations that enabled them to follow up the signs of liver and kidney toxicity revealed in Monsanto’s 90-day test on NK603 maize. Séralini also measured sex steroid hormone levels, which is not measured in industry tests on GMOs. This turned out to be an important addition, as both NK603 maize and Roundup disturbed hormone levels and function.
Hiltzik implies that Séralini set out to find tumours or cancer and simply found what he was looking for. But if this were the case, Séralini would have designed his study as a 50 animal per sex per group carcinogenicity study. There was no reason for him to do so, since no pre-existing evidence, from Monsanto or elsewhere, indicated that NK603 maize or tiny doses of Roundup caused cancer.
The increase in tumour incidence in Séralini’s study was a surprise. But Séralini followed the correct scientific procedure for such a finding. He noted and recorded details of the tumours, including size, time of onset, speed of growth, and number per animal. An example of such a procedure is described in OECD chronic toxicity protocol 453 (“lesions”, the word used in OECD 453, include tumours).
Séralini did not set out to do a full-scale carcinogenicity study, which requires 50 animals per sex per group.
Yet to dismiss Séralini’s findings of increased tumour incidence because his experiment was not a carcinogenicity study would be ethically irresponsible and scientifically unsound.
If we follow the logic of Séralini’s critics, then because his study wasn’t a carcinogenicity study, we are supposed to pretend that these tumours did not occur. But this is to deny the biological reality of the findings. Other important effects included severe kidney and liver pathology, which led to an increased mortality rate.
This logic also leads to the conclusion that industry and regulators are free to dismiss any tumours that appear in a chronic toxicity experiment and not do any further investigations. And that the product that caused the tumours can be marketed as safe.
As for claims that Séralini is biased against GMOs and that therefore his results are unreliable, it should be remembered that the studies that form the basis of authorizations of GMOs are commissioned and carried out by industry. This is a problem as scientific reviews comparing studies by industry- and publicly-funded researchers on a variety of risky products, from tobacco7 to pharmaceuticals8 and mobile phones,9 have shown that industry studies are biased in the direction of concluding that the product is safe. This has also been found to be true of studies on GMOs.10 11
It is also not clear how a supposed bias on Séralini’s part could magically make tumours and organ damage appear out of nowhere.
On the other hand, it is clear that studies can be biased in the direction of false negatives (false conclusions of safety). Studies can be designed not to see certain effects, or effects seen may not be recorded.
There is a particular risk with industry studies, which are often not peer-reviewed or published in the scientific literature. GM industry safety studies on GMOs are typically only written up for publication after authorization has been granted.3 12 13 Industry toxicity studies on pesticides are typically kept hidden under commercial confidentiality arrangements with regulators.14
References:
1. Hiltzik M. Using junk science to promote Proposition 37. Los Angeles Times. 14 October 2012. http://articles.latimes.com/2012/oct/14/business/la-fi-hiltzik-20121014/2
2. European Food Safety Authority (EFSA). Review of the Séralini et al. (2012) publication on a 2-year rodent feeding study with glyphosate formulations and GM maize NK603 as published online on 19 September 2012 in Food and Chemical Toxicology. EFSA Journal. 2012; 10(10): 2910.
3. Hammond B, Dudek R, Lemen J, Nemeth M. Results of a 13 week safety assurance study with rats fed grain from glyphosate tolerant corn. Food Chem Toxicol. Jun 2004; 42(6): 1003-1014.
4. de Vendomois JS, Roullier F, Cellier D, Séralini GE. A comparison of the effects of three GM corn varieties on mammalian health. Int J Biol Sci. 2009; 5(7): 706–726.
5. European Food Safety Authority (EFSA). Opinion of the Scientific Panel on Genetically Modified Organisms on a request from the Commission related to the safety of foods and food ingredients derived from herbicide-tolerant genetically modified maize NK603, for which a request for placing on the market was submitted under Article 4 of the Novel Food Regulation (EC) No 258/97 by Monsanto (QUESTION NO EFSA-Q-2003-002): Opinion adopted on 25 November 2003. EFSA Journal. 2003; 2003(9): 1–14.
6. Séralini GE, Clair E, Mesnage R, et al. Long term toxicity of a Roundup herbicide and a Roundup-tolerant genetically modified maize. Food and Chemical Toxicology. November 2012; 50(11): 4221-4231.
7. Barnes DE, Bero LA. Why review articles on the health effects of passive smoking reach different conclusions. JAMA. May 20 1998; 279(19): 1566-1570.
8. Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. British Medical Journal. 2003; 326: 1167.
9. Huss A, Egger M, Hug K, Huweiler-Müntener K, Röösli M. Source of funding and results of studies of health effects of mobile phone use: Systematic review of experimental studies. Environmental Health Perspectives. January 2007; 115: 1–4.
10. Diels J, Cunha M, Manaia C, Sabugosa-Madeira B, Silva M. Association of financial or professional conflict of interest to research outcomes on health risks or nutritional assessment studies of genetically modified products. Food Policy. 2011; 36: 197–203.
11. Domingo JL, Bordonaba JG. A literature review on the safety assessment of genetically modified plants. Environ Int. Feb 4 2011; 37: 734–742.
12. Hammond B, Lemen J, Dudek R, et al. Results of a 90-day safety assurance study with rats fed grain from corn rootworm-protected corn. Food Chem Toxicol. Feb 2006; 44(2): 147-160.
13. Hammond BG, Dudek R, Lemen JK, Nemeth MA. Results of a 90-day safety assurance study with rats fed grain from corn borer-protected corn. Food Chem Toxicol. Jul 2006; 44(7): 1092-1099.
14. Antoniou M, Habib MEM, Howard CV, et al. Teratogenic effects of glyphosate-based herbicides: Divergence of regulatory decisions from scientific evidence. J Environ Anal Toxicol. 2012.
Sources of criticism:
Jon Entine, director, Genetic Literacy Project, writing in Forbes magazine
European Food Safety Authority
http://www.efsa.europa.eu/en/efsajournal/pub/2910.htm
http://www.efsa.europa.eu/en/efsajournal/pub/2986.htm
Haut Conseil des Biotechnologies (HCB), France
http://www.hautconseildesbiotechnologies.fr/IMG/pdf/Etude_Seralini_Avis_CS_HCB_121019.pdf