Summary answer:
This criticism centres on the incorrect assumption that Séralini’s study is a carcinogenicity study, and concludes that it is poorly designed for this purpose. But Séralini’s study was not a carcinogenicity study, but a chronic toxicity study. Thus Séralini avoided over-interpreting the increase in tumour incidence observed and did not claim that NK603 maize or Roundup are carcinogenic in humans. Further studies must be carried out before such conclusions can be drawn. What is concluded is that NK603 maize and Roundup had serious toxic effects on rats, including kidney and liver damage, increased mortality, and the increased and earlier development of tumours, especially in female rats.
Detailed answer:
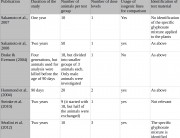
This criticism centres on the incorrect assumption that Séralini’s study is a carcinogenicity study, and concludes that it is poorly designed for this purpose. But as stated in the introduction to the paper, he had no reason to embark on a carcinogenicity study. He designed the study as a chronic toxicity study.
According to OECD and other standardized protocols for carcinogenicity studies, Séralini did not have a large enough number of rats to do a statistical analysis on the tumour or mortality findings. Therefore he did not perform a statistical analysis on those effects. Nor did he draw any conclusions regarding the carcinogenicity of NK603 maize or Roundup at the doses tested. Séralini simply noted the number, type, speed of growth, and time of onset of tumours, in line with normal scientific practice in a chronic toxicity test – for example, as stipulated for the chronic toxicity phase of OECD 453.1
Séralini did conduct a statistical analysis on biochemical measurements, for which he did have enough animals.
What can be concluded from Séralini’s experiment is that NK603 maize and Roundup had serious toxic effects on rats, including kidney and liver damage, increased mortality, and the increased incidence and earlier development of tumours, especially in female rats. The logical conclusion that must be drawn from these findings is that a full-scale carcinogenicity study using 50 animals per sex per group must be carried out on NK603 maize and Roundup.
While Séralini’s findings should not be over-interpreted, it is ethically and scientifically unacceptable for regulators to dismiss them. Such a stance inevitably raises questions as to how they would normally react to such findings in a chronic toxicity study carried out by industry in support of regulatory authorization for a product. Would regulators brush aside the findings and conclude that the product was safe to market? Or would they insist that industry follow up with a full-scale carcinogenicity study?
The public has a right to expect that regulators would follow the latter course. But if regulatory authorities treat industry studies in the same way that they treat Séralini’s – by dismissing the findings of toxicity – this implies that they would allow industry to market a product that can potentially cause increased tumours and organ damage, without further investigation. And that’s an extremely worrying prospect.
Reference:
1. Organisation for Economic Cooperation and Development (OECD). OECD guideline no. 453 for the testing of chemicals: Combined chronic toxicity/carcinogenicity: Adopted 7 September 2009. 2009.
Sources of criticism:
Science Media Centre “experts”
http://www.sciencemediacentre.org/expert-reaction-to-gm-maize-causing-tumours-in-rats/
Monsanto
http://www.monsanto.com/products/Documents/ProductSafety/seralini-sept-2012-monsanto-comments.pdf
European Food Safety Authority
http://www.efsa.europa.eu/en/efsajournal/pub/2910.htm
http://www.efsa.europa.eu/en/efsajournal/pub/2986.htm
BfR (German federal institute for risk assessment)