Summary answer:
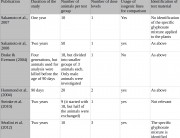
The rats in Séralini’s study had unrestricted access to food and water, but so did the rats in Monsanto’s 90-day studies on GM foods, and so do most humans in their daily lives. So this aspect of Séralini’s study reflects standard industry testing practices as well as realistic human exposures.
Detailed answer:
The rats in Séralini’s study had unrestricted access to food and water. This is also normal practice in the 90-day industry feeding studies on GM foods.1 2 3 The European Food Safety Authority (EFSA) has never questioned this with regard to industry studies. The Organisation for Economic Cooperation and Development also recommends unrestricted access to food and water in its guidelines for long-term tests.4
Most humans too do not restrict their diet, and are about as prone to tumours as SD rats, or slightly more so (see Criticism: The fact that 30% of control females got tumours shows this rat is an unreliable model). So it is difficult to see why Séralini’s critics would promote an unrealistic protocol in which diet is restricted, unless their intention was to hide the toxic effects of NK603 maize and Roundup.
Also, both treatment and control groups in Séralini’s study had unrestricted access to food. So the increase in the treatment groups was likely to be due to the substances being tested: GM NK603 maize and Roundup.
References:
1. Hammond B, Dudek R, Lemen J, Nemeth M. Results of a 13 week safety assurance study with rats fed grain from glyphosate tolerant corn. Food Chem Toxicol. Jun 2004; 42(6): 1003-1014.
2. Hammond B, Lemen J, Dudek R, et al. Results of a 90-day safety assurance study with rats fed grain from corn rootworm-protected corn. Food Chem Toxicol. Feb 2006; 44(2): 147-160.
3. Hammond BG, Dudek R, Lemen JK, Nemeth MA. Results of a 90-day safety assurance study with rats fed grain from corn borer-protected corn. Food Chem Toxicol. Jul 2006; 44(7): 1092-1099.
4. Organisation for Economic Cooperation and Development (OECD). Guidance document 116 on the conduct and design of chronic toxicity and carcinogenicity studies, supporting test guidelines 451, 452 and 453: 2nd edition: Environment directorate joint meeting of the chemicals committee and the working party on chemicals, pesticides and biotechnology. 13 April 2012.
Sources of criticism:
Science Media Centre “experts”, notably Tom Sanders
http://www.sciencemediacentre.org/expert-reaction-to-gm-maize-causing-tumours-in-rats/