The obstructive response of the European Food Safety Authority’s (EFSA) scientific committee (below) to the request of the European Commission that it help with the design of long-term carcinogenicity tests on GM feed is shameful.
Source: GM Watch
The Commission made its request to EFSA after the publication of the 2012 Seralini study, which showed that feeding a Monsanto GM maize, NK603, and tiny amounts of Roundup herbicide to rats over a long-term 2-year period had serious toxic effects, including organ damage and increased tumour rates.
The EFSA scientific committee, which includes GMO Panel head Joe Perry, “raised some concern about the usefulness of such a trial with whole foods/feeds without having a clear objective for such a trial”.
It’s hard to make sense of such institutional stupidity, except by considering the conflicts of interest with the GM industry that have plagued the members of the previous GMO Panel:
http://corporateeurope.org/sites/default/files/publications/amflora_coi_report_2011.pdf
Some of these members, including Joe Perry, have continued into the present Panel.
Let’s spell it out for EFSA’s GMO Panel and the scientific committee, who are clearly struggling to get their heads around the science.
The “objective” of a carcinogenicity test is to test the substance for carcinogenicity (cancer-causing effects). The “objective” of a long-term chronic toxicity test is to test for chronic toxicity. Seralini’s study shows that GMOs and their associated pesticides should be tested for both types of effect.
As for the committee’s statement, “An assessment of carcinogenicity/chronic toxicity should be carried out after initial information on toxicity has been obtained from repeated dose 28 and/or 90-day toxicity tests”, the whole point about Seralini’s study is that the first tumours were only seen 4 months into the trial. That’s a full one month after a 90-day toxicity test has ended.
Whatever your view of Seralini’s study, it cannot be denied that it shows that 90-day tests are too short to reliably show carcinogenic or chronic toxic effects.
Contrary to the scientific committee’s statement, you cannot “assess” carcinogenicity or chronic toxicity on the basis of the 28- or 90-day tests – except by prolonging the trial into a 2-year study. This is what Seralini did! But his results have been dismissed by EFSA.
Even the French agencies HCB and ANSES, which were otherwise critical of the study (perhaps because both agencies are responsible for national authorisations of this or other GM foods), acknowledged the need for long-term studies.
Is it unreasonable to wonder whether the reason the EFSA scientific committee doesn’t want the carcinogenicity studies that the Commission has rightly requested is because they might find worrying results?
—
—
EFSA resistance to two-year cancer study on GM feed
EU Food Policy, May 17 2013
Reproduced with kind permission of EU Food Policy: http://www.eufoodpolicy.com/
Members of EFSA’s Scientific Committee are resisting the long-term carcinogenicity study on rodents fed genetically-modified feed planned by the European Commission.
EFSA is expected to be asked to provide support for a protocol on this study and its advice would help shape the planned research project by DG Research and Innovation.
But according to the EFSA note of a meeting of the Scientific Committee, experts “raised some concern about the usefulness of such a trial with whole foods/feeds without having a clear objective for such a trial”.
“In addition, information that will assist in such a study design should take into account results of any /in vitro/ or/in vivo /toxicity tests.
“An assessment of carcinogenicity/chronic toxicity should be carried out after initial information on toxicity has been obtained from repeated dose 28 and/or 90-day toxicity tests,” they said.
The Scientific Committee is made up of all the chairs of the individual panels, including Joe Perry of the GMO panel, and other senior scientists appointed by the Authority.
















































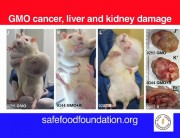

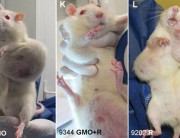


















We have more coverage of this on the GM-Free Cymru web site, here:
http://www.gmfreecymru.org/news/Press_Notice23May2013.html