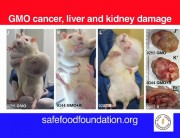
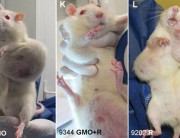
The study that showed increased mortality in rats fed GM maize has sparked an outcry and reignited the debate on the approval of GMOs and pesticides.
The findings of the controlled study of Gilles-Eric Séralini, Professor of Biology at the University of Caen, threw a stone into the pond by showing an increased risk of tumours and mortality in rats that consumed transgenic maize NK603 for almost all their lives (see protocol and results in box below) [1].
This case recalls the recent public health scandal over Servier’s Mediator [a diabetes drug marketed by Servier that caused thousands of deaths and hospitalisations and was subsequently banned], not only by questioning the process of authorising a product for the market, this time a product intended for food, but also because of the strong methodological criticisms that French toxicologists has levelled at the study.
Complaints against the authors of the study and the peer-reviewers at the journal that published it, Food and Chemical Toxicology, include poor choice of experimental model, too small sample size and low statistical power, partisan interpretation.
A recent editorial in Nature[2] states, however, that Jose Domingo (toxicologist at the University Rovira i Virgili University in Reus, Spain), editor for the journal Food and Chemical Toxicology, said that the study raised no red flags during the process of peer review.
We note that the publication of the article by Gilles-Eric Séralini was accompanied by a book written by the same author “Are we all guinea pigs?”, and a television documentary. The scientists were also accused of media hype.
Medscape.fr asked Dr Joel Spiroux de Vendomois, general practitioner and co-author of the study, and Chairman of the Committee for Independent Research and Information on Genetic Engineering (CRIIGEN), to respond to various criticisms.
Dr Spiroux responds to the criticisms
Medscape.fr: Before reviewing the criticisms, why did you choose this maize in particular?
Dr Spiroux: For several reasons. First, the most widely used GM crops in the world are those that are tolerant to Monsanto’s Roundup. Approximately 60 to 70% of GMOs on the planet have the characteristic of being tolerant to Roundup, 20% produce their own insecticide, such as Bt maize, and others, have several genes of interest, the most recent is SmartStax maize, which has 8 genes – both genes for tolerance to Roundup, and genes that produce insecticides.
The second point is that since 2008 we have published a few studies conducted to reassess toxicology studies conducted over three months by Monsanto on MON863 corn, MON810 corn and NK603 corn. We found signs of liver and kidney toxicity, which prompted us to investigate the effects of maize NK603 consumed over the long term.
Finally, corn is grown and consumed by animals and humans in the United States, Europe and other countries (authorization was granted in the United States in 2000 and later in 14 countries plus the European Union).
Medscape.fr: In parallel with the groups receiving GM maize with and without Roundup, you also gave some of the rats drinking water containing Roundup, in its complete formulation. Why?
Dr Spiroux: For the authorization of pesticides for the market, and in particular Roundup, toxicological studies are performed on what is called the “active” ingredient, in this case glyphosate – never the complete formulation. Producers can then use this active ingredient in different formulations for different purposes. They do not have to test again. However, studies published in peer-reviewed journals have shown that in vitro, glyphosate alone is 10 to 100 times less toxic than glyphosate in combination with these additives. We wanted to see how toxic it is in vivo.
Medscape.fr: Moving to the criticisms. The editorial in Nature referred to a dozen long-term studies on GMOs which did not find these harmful effects. How do you respond?
Dr Spiroux: Nature made reference to a review published in January this year by Chelsea Snell et al [3] who compiled 24 long-term studies on GMOs (see protocol and results in the text box at the end). In fact, these studies are nutritional, not toxicological studies. This is very different from what we did.
Also, these studies are called “long-term” but they are not “lifetime” like ours. One study, for example, was carried out over two years in pigs. For the moment no lifetime study has been done on mammals. This is a way to sidestep the issue. It also referred to trans-generational studies, including a Japanese study published by Sakamoto et al. conducted over 1000 days[4]. But when you look at the study in detail, the rats were only fed with GMOs for 41 days. One must be very careful when comparing these studies.
Medscape.fr: You were much criticised for your choice of rats, a line that spontaneously develops tumours, especially the females. How do you justify your choice?
Dr Spiroux: We are accused of having used a variety of rats that are more prone to tumours than others but these rats are widely used for experiments. On the one hand, I do not see why we should choose rats that have no susceptibility to developing diseases. Finally, it is for this very reason that we have control rats! If our variety of rats was no good, then the marketing authorisations for all of Monsanto’s GMOs must be cancelled because it did its studies on the same variety of rat …
Medscape.fr: You are also accused of having a too small number of rats per group …
Dr Spiroux: Again, the Monsanto seed company studied groups of the same size.
From a scientific point of view, we are told that carcinogenicity studies require 50 rats per group. My answer is that we had no a priori reason to go with the idea of a tumorigenic effect. By re-analyzing the Monsanto studies, we had uncovered signs of liver and kidney toxicity. If we had thought that there could be a tumorigenic effect from the beginning, we would not have studied as many groups and we would have had groups with a larger number of rats.
OECD protocol No. 408: The study follows this standard
The study protocol for assessing the toxicity of chemicals in Europe, OECD No. 408, recommends populations of at least 10 animals per sex per group with three doses of the test substance and a control group. That protocol was observed in the study by Gilles-Eric Seralini.
Medscape.fr: Moreover, there was no dose-response effect. Does this discredit your results?
Dr Spiroux: It is possible to have a stimulating effect with small doses and saturating effects with higher doses. We know already that there is no dose-response effect with endocrine disruptors. The endocrine system does not respond directly in a dose-dependent way.
Medscape.fr: Another criticism: No health effects have been directly associated with GMOs in humans even though Americans have been eating them for 15 years …
Dr Spiroux: For 50 years, there has been an increase in endocrine disorders, infertility, cancer, decreased sperm count, neurodegenerative diseases, birth defects … However, we observe that these conditions also develop in wildlife and are therefore probably caused by environmental factors.
In the United States, if diseases were triggered by pesticides and GMOs, it would be impossible to make a direct link as products are not labeled and are mixed with a toxic soup that we have already in our blood.
Remember also that even if alcohol, tobacco, drugs and food are clearly identified as causative agents of cancer, many cancers remain unexplained. Epidemiology is not adapted to environmental diseases. A combination of disease-causing substances can produce X different pathogenic manifestations depending on the individual, doses, and time of exposure. We do not find the binary relationship which is expected with classical epidemiology, which has been designed to monitor epidemics associated with a bacterial causal agent and also works for gross toxins like mercury, cadmium, lead … For finer elements one must change the paradigm. That there we see no binary relationship (here) does not mean that it does not exist. These are complex issues.
It will also be necessary to review toxicology, which is based on dose-response effects, on identical effects in women and men, and [on no differentiation according to] period of life.
Criticisms based on the lack of dose-response effect, on differences between males and females, as well as the evaluation criterion that consists of the absence of histopathological changes after three months do not hold, in my opinion.
You have to work on the toxicology of low doses and combined effects of toxic substances.
Medscape.fr: The European Food Safety Authority (EFSA), The French Agency for Food, Environmental and Occupational Health Safety (ANSES) and the High Council of Biotechnology (HCB) will evaluate your results. Do you favour such counter-expertise?
Dr Spiroux: As President of CRIIGEN and co-author of this study, I am for contradictory studies. But right now we are not ready to give our results as long as the State and Europe do not publish all toxicology studies that allowed assessment bodies to authorize the placing on the market of GMOs or pesticides. Here, we point the finger at the intellectual scam that took place in Europe for 50 years on the method of evaluation of these chemicals.
Medscape.fr: What can doctors tell their patients about the issue of long-term health effects of GMOs?
Dr Spiroux: The medical fraternity is set up for care, not prevention. This concept of the relationship between health and the environment is not really in their minds. At all costs, environmental health issues should be included in the medical curriculum, there should be a chair of environmental health in all medical schools, and there should be continuing medical education on environmental pathologies.
[Medscape.fr:] Should protocols for market authorization be reviewed?
Whatever one thinks of this new study, it has the merit of reopening the debate on the safety of GM foods and herbicides in food.
And if scientists are reacting strongly, so are politicians. In France, Prime Minister Jean-Marc Ayrault announced that if the danger of certain GMOs was verified, France would “defend at European level” its ban GM maize NK603. As for Russia, it has temporarily suspended the import and marketing of GM maize NK603.
This debate is not new and, until recently, in February 2011, ANSES published a report in which it stated that “there are certain shortcomings in the tests currently required at European level in the context of evaluating toxicity of GMOs.”
This report noted “margins of error inherent in the implementation of subchronic toxicity studies administration of the plant via food for 90 days in rats, as established by OECD protocol No. 408 and requested by the European Food Safety Authority (EFSA) in the assessment of GMOs prior to their being placed on the market.” According to the authors, “the [statistical] power is inadequate in 50% and 80% of the tests respectively.” [? “la puissance est insuffisante dans respectivement 50 % et 80 % des tests]
Following the publication of Séralini et al.’s study, Minister of Agriculture Stephane Le Foll, for his part, felt that it was necessary to revise the protocol for GMO authorization at EU level, whether or not the study is validated by scientists.
“We must rethink how our society evaluates chemicals before putting them on the market. It is an economic problem, certainly, but also an ethical one,” says Dr Spiroux.
To be continued …
Protocol and main findings of the study by Seralini et al.
The study was conducted on 200 rats of the Sprague-Dawley strain (100 of each sex) divided into 20 groups of 10 rats for two years.
3 groups of male and 3 female groups received different concentrations of GM maize in proportions of 11%, 22% or 33% of the diet, in the form of nuggets throughout the duration of the study;
3 groups of males and 3 female groups were fed the GM maize 11%, 22% or 33%, treated during cultivation with the herbicide Roundup (Monsanto);
3 groups of males and 3 female groups were fed non-transgenic maize but received varying concentrations of Roundup diluted in water (50 ng/l, 400 mg/l, 2.25g/l);
A control group of 10 male rats and a control group of 10 female rats were fed with non-transgenic maize.
The researchers conducted a mortality study of anatomical pathology, and analysis of biochemical parameters.
Control males survived an average of 624 days, control females averaged 701 days. In the control group, 3 males (30%) and 2 females (20%) died.
In groups fed GM maize, 50% of males and 70% females died prematurely, before the average life expectancy. Mortality rates were not dose-dependent on the concentration of genetically modified maize in the diet.
As early as 24 months, that is to say at the end of their lives, from 50% to 80% of females fed GMOs had developed tumours (fibroadenomas and keratoacanthomas) as against only 30% fed the GMO-free diet.
In females, mammary tumours (93% of tumours) and pituitary tumours developed earlier than in the control rats.
In males, the majority died of liver or kidney problems. “We found progressive chronic kidney disease in greater numbers in rats fed GM corn, especially in the males,” says Dr Spiroux, co-author of the study.
Males who received GM maize did not have more tumours than controls. However, the authors state that in the three groups of males who received the transgenic maize, tumours, or kidney and liver pathologies appeared as early as the 4th month and exploded in the 11th and 12th months. For the control group, the tumours occurred mainly at the end of life, in the 23rd month and 24 months.
The authors state that the results are similar in terms of tumour incidence and mortality in animals fed NK603 without Roundup and those fed NK603 with Roundup.
Study by Chelsea Snell et al: a quarter of studies had an insufficient number of animals
The literature review by Chelsea Snell et al. included 24 studies, all carried out by public laboratories, on lines of genetically modified maize, potato, soybean, rice and triticale (wheat-rye hybrid):
– 12 long-term studies longer than 90 days conventionally used in toxicity tests on GMOs (under 2 years)
– 12 studies on several generations of animals.
They showed no health problems associated with long-term consumption of food derived from GMOs.
The authors conclude: “Studies of more than 90 days, and multi-generational studies showed no effect that has not been shown in studies of 90 days. It can therefore be assumed that the standard protocols [OECD No. 408, 1998, 90 days] are sufficient to detect side effects and it is not necessary to create new protocols that do not provide supplementary information.”
However, the authors note that “the major biases observed in some papers emphasize the urgent need to improve the review process prior to publication of results on this topic.”
And they noted that only 6 of the 24 studies they reviewed used an appropriate number of animals.
Dr Joel Spiroux de Vendomois is a general practitioner, qualified in acupuncture, homeopathy, osteopathy, human ecology and traditional Chinese medicine. He is Chairman of CRIIGEN (Committee for Independent Research and Information on Genetic Engineering). It was created in 2005 in Rouen, at the first French national congress on environmental pathologies.
Jose Domingo has published criticisms of the methods of assessment of GMOs.
References
1. Seralini,G.-E. et al.Food Chem. Toxicol. http://dx.doi.org/10.1016/j.fct.2012.08.005(2012).
2. Butler. D. Tumours in rats fed genetically modified maize developed more readily than in controls, recent research reports. Nature Volume: 489, p484. 27 September 2012
3. Chelsea S., Bernheim A, Bergé JB, Kuntz M, et al. Food and Chemical Toxicology. Assessment of the Health Impact of GM Plant Diets in Long-Term and Multigenerational Animal Feeding Trials: a Literature Review Food and Chemical Toxicology 50 (2012) 1134-1148
4. Sakamoto,Y. et al. 2008. A 104-week feeding study of genetically modified soybeans in F344rats. J.Food Hyg. Soc. Japan 49, 272-282. [Article in Japanese].
Aude Lecrubier
Holds a Master’s in Molecular Biology (Paris VI) and a Master of Scientific and Medical Journalism (New York University). Aude Lecrubier has written for over 10 years for the French and English-language press (Reuters Health, Embo Reports … ). She made her first steps in journalism at Popular Science, Science & Environment and Life magazine before devoting herself to medicine. After eight years in groups NHA Impact Communication and Medicine, she joined our team. Aude Lecrubier has no conflict of interest to declare.
Source: http://www.medscape.fr/oncologie/articles/1452135/ (Translated into English by GMWatch)