Professor Gilles-Eric Séralini and his team have responded to the letter from A. Wallace Hayes, editor of Food and Chemical Toxicology (FCT), telling Prof Séralini that he intended to retract his study on NK603 maize and Roundup.
Here’s the retraction notice from Elsevier, the publisher of FCT: http://prn.to/1euTk2W
Response by Prof GE Seralini and colleagues to A. Wallace Hayes, editor of Food and Chemical Toxicology
28 Nov 2013
We, authors of the paper published in FCT more than one year ago on the effects of Roundup and a Roundup-tolerant GMO (Séralini et al., 2012), and having answered to critics in the same journal (Séralini et al., 2013), do not accept as scientifically sound the debate on the fact that these papers are inconclusive because of the rat strain or the number of rats used. We maintain our conclusions. We already published some answers to the same critics in your Journal, which have not been answered (Séralini et al., 2013).
Rat strain
The same strain is used by the US national toxicology program to study the carcinogenicity and the chronic toxicity of chemicals (King-Herbert et al., 2010). Sprague Dawley rats are used routinely in such studies for toxicological and tumour-inducing effects, including those 90-day studies by Monsanto as basis for the approval of NK603 maize and other GM crops (Sprague Dawley rats did not came from Harlan but from Charles-River) (Hammond et al., 2004; Hammond et al., 2006a; Hammond et al., 2006b).
A brief, quick and still preliminary literature search of peer-reviewed journals revealed that Sprague Dawley rats were used in 36-month studies by (Voss et al., 2005) or in 24-month studies by (Hack et al., 1995), (Minardi et al., 2002), (Klimisch et al., 1997), (Gamez et al., 2007).Some of these studies have been published in Food and Chemical Toxicology.
Number of rats, OECD guidelines
OECD guidelines (408 for 90 day study, 452 chronic toxicity and 453 combined carcinogenicity/chronic toxicity study) always asked for 20 animals per group (both in 1981 and 2009 guidelines) although the measurement of biochemical parameters can be performed on 10 rats, as indicated. We did not perform a carcinogenesis study, which would not have been adopted at first, but a long-term chronic full study, 10 rats are sufficient for that at a biochemical level according to norms and we have measured such a number of parameters! The disturbance of sexual hormones or other parameters are sufficient in themselves in our case to interpret a serious effect after one year. The OPLS-DA statistical method we published is one of the best adapted. For tumours and deaths, the chronology and number of tumours per animal have to be taken into account. Any sign should be regarded as important for a real risk study. Monsanto itself measured only 10 rats of the same strain per group on 20 to conclude that the same GM maize was safe after 3 months (Hammond et al., 2004).
The statistical analysis should not be done with historical data first, the comparison is falsified, thus 50 rats per group is useless
The use of historical data falsifies health risk assessments because the diet is contaminated by dibenzo-p-dioxins and dibenzofurans (Schecter et al., 1996), mercury (Weiss et al., 2005), cadmium and chromium among other heavy metals in a range of doses that altered mouse liver and lung gene expression and confounds genomic analyses (Kozul et al., 2008). They also contained pesticides or plasticizers released by cages or from water sources (Howdeshell et al., 2003). Historical data also come from rats potentially fed on GMOs, some animal pellets in the world do indicate that. All that corresponds to the contamination levels for which we have detected some effects in our treated rats versus appropriate controls.
2-year historical data mammary fibroadenoma rate from Charles River SD females ranged from 13 to 62% (Giknis, 2004). We obtain a lot less in our controls, the real comparators, a lot more in treated rats. This makes our results significant, like for deaths.
Double standards
A factual comparative analysis of the rat feeding trial by the Séralini’s group and the Monsanto trials clearly reveals that if the Séralini experiments are considered to be insufficient to demonstrate harm, logically, it must be the same for those carried out by Monsanto to prove safety. Basically, all previous studies finding adverse effects of GE crops have been treated by regulators with the attitude: only those studies showing adverse effects receive a rigorous evaluation of their experimental and statistical methods, while those that claim proof of safety are taken at face value. All studies that reported no adverse effects were accepted as proof of safety regardless of these manifest (but deemed irrelevant) deficiencies of their methods.
The review by (Snell et al., 2012) illustrates this issue. In the abstract, the authors state “Results from all the 24 studies [reviewed] do not suggest any health hazards […]” – taking all those studies at face value. Yet in their review, the authors find numerous weaknesses of similar or greater severity [than those] raised for the Séralini group’s paper. For example, of the 24 studies they evaluated 16 (67% of all studies) did not mention using the isogenic line as control (interpreted as having not used them), many did not describe the methods in any detail, and according to the reviewers had other deficiencies too.
FCT should retract the Hammond et al. paper on Roundup tolerant maize for all these reasons, published for Monsanto’s authorization, or consider that each of these papers is part of the scientific debate.
References
Gamez, R., Noa, M., Mas, R., Mendoza, N., Pardo, B., Menendez, R., Perez, Y., Gonzalez, R.M., Gutierrez, A., Marrero, G., Goicochea, E., Garcia, H., Curveco, D., 2007. Long-term carcinogenicity of D-003, a mixture of high molecular weight acids from sugarcane wax, in Sprague Dawley rats: a 24 months study. Food Chem Toxicol 45, 2352-2358.
Giknis, M.L.A.a.C., C.B., 2004. Charles River Laboratories. Compilation of spontaneous neoplastic lesions and survival in Crl:CD (SD) rats from control groups.
Hack, R., Ebert, E., Leist, K.H., 1995. Chronic toxicity and carcinogenicity studies with the insecticide endosulfan in rats and mice. Food Chem Toxicol 33, 941-950.
Hammond, B., Dudek, R., Lemen, J., Nemeth, M., 2004. Results of a 13 week safety assurance study with rats fed grain from glyphosate tolerant corn. Food Chem Toxicol 42, 1003-1014.
Hammond, B., Lemen, J., Dudek, R., Ward, D., Jiang, C., Nemeth, M., Burns, J., 2006a. Results of a 90-day safety assurance study with rats fed grain from corn rootworm-protected corn. Food Chem Toxicol 44, 147-160.
Hammond, B.G., Dudek, R., Lemen, J.K., Nemeth, M.A., 2006b. Results of a 90-day safety assurance study with rats fed grain from corn borer-protected corn. Food Chem Toxicol 44, 1092-1099.
Howdeshell, K.L., Peterman, P.H., Judy, B.M., Taylor, J.A., Orazio, C.E., Ruhlen, R.L., Vom Saal, F.S., Welshons, W.V., 2003. Bisphenol A is released from used polycarbonate animal cages into water at room temperature. Environ Health Perspect 111, 1180-1187.
King-Herbert, A.P., Sills, R.C., Bucher, J.R., 2010. Commentary: update on animal models for NTP studies. Toxicol Pathol 38, 180-181.
Klimisch, H.J., Deckardt, K., Gembardt, C., Hildebrand, B., Kuttler, K., Roe, F.J., 1997. Long-term inhalation toxicity of N-vinylpyrrolidone-2 vapours. Studies in rats. Food Chem Toxicol 35, 1041-1060.
Kozul, C.D., Nomikos, A.P., Hampton, T.H., Warnke, L.A., Gosse, J.A., Davey, J.C., Thorpe, J.E., Jackson, B.P., Ihnat, M.A., Hamilton, J.W., 2008. Laboratory diet profoundly alters gene expression and confounds genomic analysis in mouse liver and lung. Chem Biol Interact 173, 129-140.
Minardi, F., Belpoggi, F., Soffritti, M., Ciliberti, A., Lauriola, M., Cattin, E., Maltoni, C., 2002. Results of long-term carcinogenicity bioassay on vinyl acetate monomer in Sprague-Dawley rats. Ann N Y Acad Sci 982, 106-122.
Séralini, G.E., Clair, E., Mesnage, R. Gress, S., Defarge, N. Malatesta, M. Hennequin, D. Spiroux de Vendômois, J. (2012) Long term toxicity of a Roundup herbicide and a Roundup-tolerant genetically modified maize. Food and Chem. Tox. 50:4221-4231
Séralini, G.E., Mesnage, R., Defarge, N., Gress, S., Hennequin, D., Clair, E., Malatesta, M., Spiroux de Vendômois, J. (2013) Answers to critics: why there is a long term toxicity due to NK603 Roundup-tolerant genetically modified maize and to a Roundup herbicide. Food and Chem. Tox. 53:461-468
Schecter, A.J., Olson, J., Papke, O., 1996. Exposure of laboratory animals to polychlorinated dibenzodioxins and polychlorinated dibenzofurans from commerical rodent chow. Chemosphere 32, 501-508.
Snell, C., Bernheim, A., Berge, J.B., Kuntz, M., Pascal, G., Paris, A., Ricroch, A.E., 2012. Assessment of the health impact of GM plant diets in long-term and multigenerational animal feeding trials: a literature review. Food Chem Toxicol 50, 1134-1148.
Voss, C., Zerban, H., Bannasch, P., Berger, M.R., 2005. Lifelong exposure to di-(2-ethylhexyl)-phthalate induces tumors in liver and testes of Sprague-Dawley rats. Toxicology 206, 359-371.
Weiss, B., Stern, S., Cernichiari, E., Gelein, R., 2005. Methylmercury contamination of laboratory animal diets. Environ Health Perspect 113, 1120-1122.
















































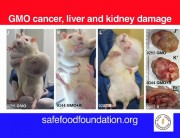

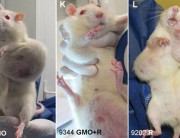


















In the coming months and years, the evidence will speak very unambiguously about the danger of Monsanto’s GMO pseudo science. Science, real Science, cannot be covered up forever — even by Monsanto and its innumerable advocates, lobbyists, and shills in Washington.
Like many people, I hope scientists and governments throughout the world will begin (or continue) to conduct rigorous scientific investigation of all GMOs and their various deadly inputs. All findings must be made public; the current cult of secrecy regarding “proprietary information” must end. Nothing short of full disclosure is to be tolerated.
The American People have been poisoned for many decades — and this has been known especially since the publication of Rachel Carson’s Silent Spring in 1962. Our soils have been destroyed by chemical agriculture, much of our water is polluted, and most American food is simply inedible — thanks to a Corporate America and Washington DC.
If we should survive for a few more decades or even centuries, I believe that our descendants will look back upon us and see 20th- and 21st- century American agriculture to be one of the biggest “scientific” and technological frauds of all time.
Please go this website and place your comment: http://pompeo.house.gov/news/documentsingle.aspx?DocumentID=398327
The above responses by Dr. Seralini are sound. I have read the study, and did not note any improper methods nor any implausible conclusions. Even so, it’s science, and can never be perfect. Still, people are always making decisions with incomplete information in life, and in this case it seems clear that erring on the side of caution would have been the sensible strategy, before unleashing these organisms on our children as well as into the ecosystems that are currently struggling to support all of our absurd habits.
Here is the letter I sent to Dr. Hayes at Elsevier at the link I provided as “my” website (Note: This is not Elsevier’s first integrity failing) — feel free to email your thoughts to Elsevier
Greetings Dr. Hayes,
As it happens my doctorate is in the field of Atmospheric Science, where the corporate influence has largely resulted in the detraction of our findings in the public sphere, rather than in the retraction of viable studies in the scientific literature.
I do hope that your journal will let the Seralini GMO article in question stand, to preserve not only the credibility of your scientific field but also the legacy of the journal itself.
If the paper’s findings are indeed so egregious, surely it will languish with all the rest of the later-proven-unsound articles in the scientific literature – unread and uncited.
If the paper’s findings are later proven to be accurate, then your journal would be redeemed to a considerable extent by having stood against the corporate leviathans which seek, in pursuit of profit, to undermine the integrity of science.
Further, I strongly suggest that, to avoid the appearance of impropriety, Elsevier install a 2-year waiting period before the upper echelon advisors, paid or unpaid, are allowed to cycle either to or from industry positions.
Kirstie Stramler, PhD, Columbia University 2006
This man is to be commended and a retraction only proves again how science is being suppressed in the interest of big corporations. Science becomes meaningless when terms like “substantially equivalent are used and then patents are slapped on a product. Is it the same or is it different? Anyone the opposes this science is to be commended as it is obvious to some of us that there are serious consequences that have become evident. To say that 3 trillion meals have been consumed without any harm also shows the lack of “science”…..how has this been proven? It is a ridiculous argument! Monsanto and the like have truly invented pseudo-science. Tobacco did the same thing and now no one would now say that lung cancer and heart disease are not associated with it’s use. This technology has extremely serious consequences. Independent studies need to be done on all products BEFORE their introduction into our environment. Industry should not have ANY say about whether a product is allowed to be produced. This is a serious conflict of interest that has been allowed for too long.
I want to thank you for your years of dedication in this field, your dedication to honest research and your independence in assessing the safety risks of GMO foods as associated pesticides.
When the article on the retraction in the Toxicology journal mentioned nothing about your qualifications in GMO foods and herbicides a whole bunch of red flags went off. I had the gut feeling that you are a leader in your field and I am pleased to see that with a little research, I was right again. There is probably a very interesting story behind the retraction that needs to be told. My assessment of David Spiegelhalter is one of a condescending, ruling class baffone.
If it is any consolation, this article on the retraction of your paper in the Reed Elsevier’s Food and Chemical Toxicology journal has introduced me to your work and expertise. I have no doubt that your research can not even be duplicated in the US because of restrictions placed on legal uses of these Monsanto products. I believe that you are required by law to notify Monsanto prior to any product research for approval.
Thank you and your staff again for providing honest research to the global community on risks associated with GMO foods, pesticides and herbicides associated with that industry.
Sincerely, Jay Lindberg