Séralini’s study shows that the current regulatory system for GMOs is inadequate. Long-term tests must be required on all GMOs before they can be commercialized.
This conclusion is shared even by organizations that have criticized Séralini’s study, such as the food safety agency ANSES and the Haut Conseil des Biotechnologies (HCB), both in France.[1, 2]
Both organizations were involved in issuing opinions that led to the authorization of NK603 maize and other GMOs in France. So they are not disinterested parties and their criticism of Séralini’s findings could be seen as defending their own authorizations.
Séralini’s study also challenges the regulatory authorizations of pesticides like Roundup. The long-term studies performed by industry on its pesticides for regulatory authorizations only test the main active ingredient: in the case of Roundup, that’s the chemical glyphosate. But research shows that the complete formulations are more toxic than glyphosate alone.[3, 4, 5]
In contrast, Séralini’s study tested the complete formulation of Roundup as it is sold and used, at levels deemed safe in drinking water and some food and feed crops. The study found that these levels still caused in increase in mortality, tumours, and organ damage.
The long-term industry tests on glyphosate performed in support of regulatory authorization are kept secret under commercial confidentiality agreements between industry and regulators.[6] Séralini is calling for the data from these studies to be made public and opened to independent scientific scrutiny.
All that is in the public domain is the German government authorities’ assessment of these industry studies on glyphosate. This assessment gives serious cause for concern. Serious toxic effects, such as birth defects, were found but dismissed by the German authorities.[6]
References:
1. ANSES (French Agency for Food Environmental and Occupational Health & Safety). ANSES highlights the weaknesses of the study by Séralini et al, but recommends new research on the long-term effects of GMOs. 22 October 2012. http://www.anses.fr/cgi-bin/countdocs.cgi?Documents/BIOT2012sa0227.pdf
2. Haut Conseil des Biotechnologies Comité Scientifique (France). Avis en réponse à la saisine1 du 24 septembre 2012 relative à l’article de Séralini et al (Food and Chemical Toxicology, 2012). 19 October 2012. http://www.hautconseildesbiotechnologies.fr/
3. Dallegrave E, Mantese FDG, Dalsenter PR, Langeloh A. Acute oral toxicity of glyphosate in Wistar rats. Online J Vet Res. 2002; 1: 29–36.
4. Richard S, Moslemi S, Sipahutar H, Benachour N, Seralini GE. Differential effects of glyphosate and roundup on human placental cells and aromatase. Environ Health Perspect. Jun 2005; 113(6): 716-720.
5. Mesnage R, Bernay B, Seralini GE. Ethoxylated adjuvants of glyphosate-based herbicides are active principles of human cell toxicity. Toxicology. Sep 21 2012.
6. Antoniou M, Habib MEM, Howard CV, et al. Teratogenic effects of glyphosate-based herbicides: Divergence of regulatory decisions from scientific evidence. J Environ Anal Toxicol. 2012.
















































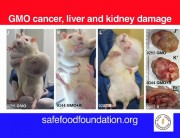

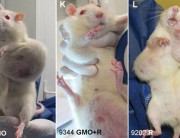






















I think the research must also involve the use of atrazine and other related chemicals, as well as 2,4-D which is very common in my country, Philippines. Its imperative that people be made aware of the truth behind the effects of the use of unproven and untested technology. Because in the Philippines it is almost impossible to test new technologies because facilities are limited and the government is not willing to do so.