Séralini designed his 2012 study as a direct followup of a previous study on the same NK603 maize conducted by Monsanto to support its application for regulatory authorization.
Monsanto’s study was a 90-day rat feeding trial on NK603. Monsanto published the results of its test in 2004,1 the same year that the maize was authorized in the EU. Differences were found in the GM-fed rats, but the European Food Safety Authority (EFSA) claimed that the differences were “of no biological significance” and that the maize was as safe as non-GM maize.2
Séralini’s team obtained Monsanto’s raw data and re-analyzed it. They found signs of liver and kidney toxicity in the GM-fed rats, publishing their findings in a peer-reviewed journal in 2009.3
Séralini carried out his 2012 study on NK603 maize and Roundup to see whether these initial findings of potential toxicity really were of no biological significance, as EFSA claimed, or whether they developed into more serious disease.4
The overall experimental design was similar to Monsanto’s, in order to make the two experiments comparable. The differences were that Séralini’s experiment was longer (two years to Monsanto’s 90 days) and far more detailed in scope. Séralini’s experiment measured a larger number of health effects and was designed to separate out the effects of the GM maize from those of the herbicide it is engineered to tolerate, Roundup. This was the first study on a GM crop to distinguish effects in this way.
The findings
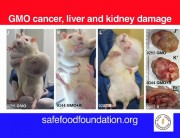
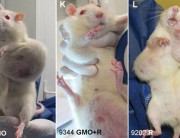
Séralini’s findings were alarming: both GM maize NK603 and Roundup caused serious kidney and liver damage and an increased and earlier development of tumours, leading to an increased rate of mortality.4
These serious effects had not shown up in Monsanto’s 90-day test because it was too short. Serious diseases like organ damage and tumours take time to develop and become obvious.
An objective analysis of Séralini’s study would conclude that long-term chronic toxicity and carcinogenicity studies are needed on all GM foods before they are commercialized.
What does the study say about the way GM foods are regulated?
Séralini’s is the first long-term peer-reviewed toxicity study on the health impacts of GM NK603 maize and the commercial herbicide formulation it is engineered to be grown with.
GM food crops are authorized on the basis of short (a maximum of 90-days) feeding studies, usually carried out in rats. Long-term studies are not required by regulators anywhere in the world.
But in Séralini’s study the first large tumours were only seen four months into the trial in the case of males and seven months in the case of females. Most tumours were only detected after 18 months.
This shows that the 90-day tests routinely done on GM crops are not long enough to detect serious health effects that take time to develop, such as cancer and organ damage.
Ninety days in a rat is equivalent to only 7–9 years in human terms5 – yet human beings could eat a GM food and residues of Roundup over a lifetime.
References:
1. Hammond B, Dudek R, Lemen J, Nemeth M. Results of a 13 week safety assurance study with rats fed grain from glyphosate tolerant corn. Food Chem Toxicol. Jun 2004; 42(6): 1003-1014.
2. European Food Safety Authority (EFSA). Opinion of the Scientific Panel on Genetically Modified Organisms on a request from the Commission related to the safety of foods and food ingredients derived from herbicide-tolerant genetically modified maize NK603, for which a request for placing on the market was submitted under Article 4 of the Novel Food Regulation (EC) No 258/97 by Monsanto (QUESTION NO EFSA-Q-2003-002): Opinion adopted on 25 November 2003. EFSA Journal. 2003; 2003(9): 1–14.
3. de Vendomois JS, Roullier F, Cellier D, Séralini GE. A comparison of the effects of three GM corn varieties on mammalian health. Int J Biol Sci. 2009; 5(7): 706–726.
4. Séralini GE, Clair E, Mesnage R, et al. Long term toxicity of a Roundup herbicide and a Roundup-tolerant genetically modified maize. Food and Chemical Toxicology. November 2012; 50(11): 4221-4231.
5. Soffritti M, Belpoggi F, Degli Esposti D. Cancer prevention: The lesson from the lab. In: Biasco G, Tanneberger S, eds. Cancer Medicine at the Dawn of the 21st Century: The view from Bologna. Bologna: Bononia University Press; 2006:49–64.




































































I am forever grateful for Dr. Seralini and his team for braving the corporate and industrial storm of lies and to have done an independent study on GM food safety. Eventually, more of these tests will produce the same results and regulatory agencies will have to respond. Meanwhile, smart and educated consumers will forge a path here in the U.S.A and hopefully get these toxic, dangerous foods labeled.
I so appreciate your dedication to the science of GM foods to bring awareness to the public. You already have made a huge difference in the U.S.
Connecticut recently held its first hearing with the Public Health Committee on a bill to label GMO foods. A Monsanto representative, in opposition to the bill, made reference to Dr. Seralini’s fraudulent research findings . Unfortunately for the Monsanto representative, Dr Michael Hansen, Senior Scientist, Consumers Union, soon after took the microphone and quickly debunked every one of Monsanto’s claims and cited recent conclusive findings that Seralini’s results were indeed accurate.
I might add that while Dr. Hansen offered the Committee documentation verifying his positions, the Monsanto and other Biotechnology representatives offered no documentation in support of theirs.
Reading about Seralini’s work caused me to go back and read the 2002 Monsanto rat study. It states clearly that of all the 40 rats that were examined microscopically were “remarkable”. All had damage, some quite significant! Livers, kidneys and spleens seemed to show consistent damage.
http://web.archive.org/web/20051109175625/http://www.monsanto.com/monsanto/content/sci_tech/prod_safety/fullratstudy.pdf